Abstract
Background: The bone marrow microenvironment (BMME), including mesenchymal stromal cells (MSCs) and osteolineage cells, vascular endothelial growth factor (VEGF) signaling, and marrow angiogenesis, is altered in myelodysplastic syndromes (MDS). BMME manipulation represents a novel therapeutic strategy for MDS. Preclinical studies have demonstrated that parathyroid hormone (PTH) increases MSC and osteoblast support of hematopoietic stem cells and remodels marrow vascularity. This effect can be augmented with the addition of bevacizumab (BEV), which also decreases angiogenesis. Here we report the results of a phase 1 study of abaloparatide (ABL), a PTH-related protein analog, plus BEV in patients with MDS.
Methods: Eligible patients were ≥ age 18 with MDS or non-proliferative chronic myelomonocytic leukemia and at least one cytopenia. Those categorized as very-low to intermediate risk per the Revised International Prognostic Scoring System (IPSS-R) who were treatment-naive or had received only prior growth factors were eligible. Patients of all risk categories who had not responded to disease modifying agents were also eligible. Prior therapies were stopped at least 14-days before study start. Patients received one 28-day cycle of ABL (80 mcg/day self-injected) followed by three 28-day cycles of ABL + BEV (5 mg/kg on days 1 and 15). Bone marrow samples were collected at baseline, after cycles 1 and 4, and 3 months after treatment completion. The primary endpoint was the rate of treatment-limiting toxicities (TLTs). Response rates, impact of therapy on BMME cell populations, and effect on health-related quality of life (HRQoL) and geriatric assessment were additional endpoints.
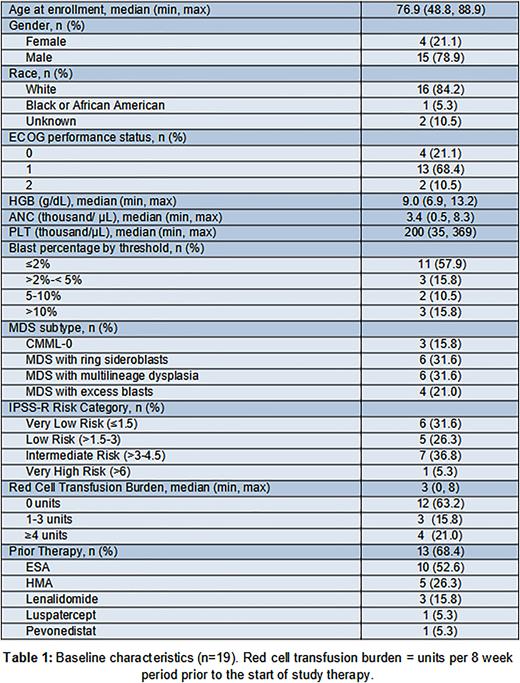
Results: Nineteen patients were enrolled and treated. The majority (n=13) received prior therapy, most commonly with an erythropoietin stimulating agent (ESA; 77%). Typical molecular heterogeneity was present and included mutations in SF3B1 (44%), TET2 (33%), ASXL1 (22%), and DNMT3A (22%). No TP53 mutations were noted. Additional baseline characteristics are outlined in Table 1. Three patients (16%) experienced a TLT: a grade 3 infusion-related reaction and grade 2 fatigue with orthostatic hypotension attributed to ABL, and a grade 3 colonic perforation possibly attributed to BEV. There were no other grade 3 events related to treatment (TRAEs). Frequent (≥15%) TRAEs were: back pain (n=6), chills (n=3), and epistaxis (n=3). Analysis of bone marrow samples revealed a significant increase in mean MSC colony forming units (CFU-Fs) after cycle 1 of ABL compared to baseline (20.7 ± 5.8 vs 36.5 ± 6.4 CFU-F per 1x106 cells, p=0.004). This increase was not maintained with the addition of BEV.
Of the 17 patients evaluable after at least 2 cycles on study, 15 (88%) had stable disease and 2 (12%) had progression. Five of 6 patients (83%) with MDS with excess blasts 2, prior hypomethylating agents, and/or high to very-high IPSS-R maintained stable disease over 7 months of follow up. The sixth patient achieved a neutrophil response by 2006 International Working Group criteria after 8 weeks of therapy. In the subset of patients with baseline neutropenia (n=4) there was a significant increase in absolute neutrophil count (ANC) after 8 weeks on study (0.89 ± 0.26 vs 1.28 ± 0.32 103 cells/µL, p=0.04). All patients with baseline neutropenia or thrombocytopenia that had improved ANC (n=4) or platelet counts (n=3), respectively, over the first 8 weeks of treatment, had increased CFU-Fs after cycle 1. Three patients (18%) had decreased transfusion needs over the first 8 weeks despite discontinuation of prior ESAs; all 3 also demonstrated increases in CFU-Fs after cycle 1. HRQoL and geriatric assessments were stable throughout the study.
Conclusions: ABL + BEV therapy is safe and well-tolerated in MDS. A significant increase in MSCs was noted following single-agent ABL for 4 weeks, suggesting BMME remodeling. This corresponded with significant early improvements in ANC values and transfusion requirements during the first 8 weeks of therapy in some patients. Prolonged stable disease was maintained in a majority of patients, including several with high-risk clinical features. These data suggest successful manipulation of the BMME with effects on hematopoiesis, and warrant further investigation of longer ABL monotherapy as a new treatment paradigm for patients with MDS.
Disclosures
Loh:Seattle Genetics: Consultancy; Pzifer: Consultancy, Honoraria. O'Dwyer:BEAM Therapeutics: Consultancy. Akwaa:Sobi: Research Funding. Liesveld:Blueprint Sciences: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; Servier: Membership on an entity's Board of Directors or advisory committees; Pharmacosmos: Membership on an entity's Board of Directors or advisory committees; SYROS: Other: DSMB. Calvi:Massachusetts General Hospital and Harvard Medical School: Patents & Royalties: U.S. Patent No. 8,802,104 B2; University of Rochester School of Medicine and Dentistry: Patents & Royalties: U.S. Patent No. 9,394,520.
OffLabel Disclosure:
Abaloparatide - a parathyroid hormone related protein analog FDA approved for the treatment of postmenopausal osteoporosis. Bevacizumab - a vascular endothelial growth factor inhibitor FDA approved for the treatment of colorectal cancer, non-small cell lung cancer, glioblastoma, renal cell carcinoma, hepatocellular carcinoma, cervical cancer, ovarian, fallopian tube, and primary peritoneal cancer.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal